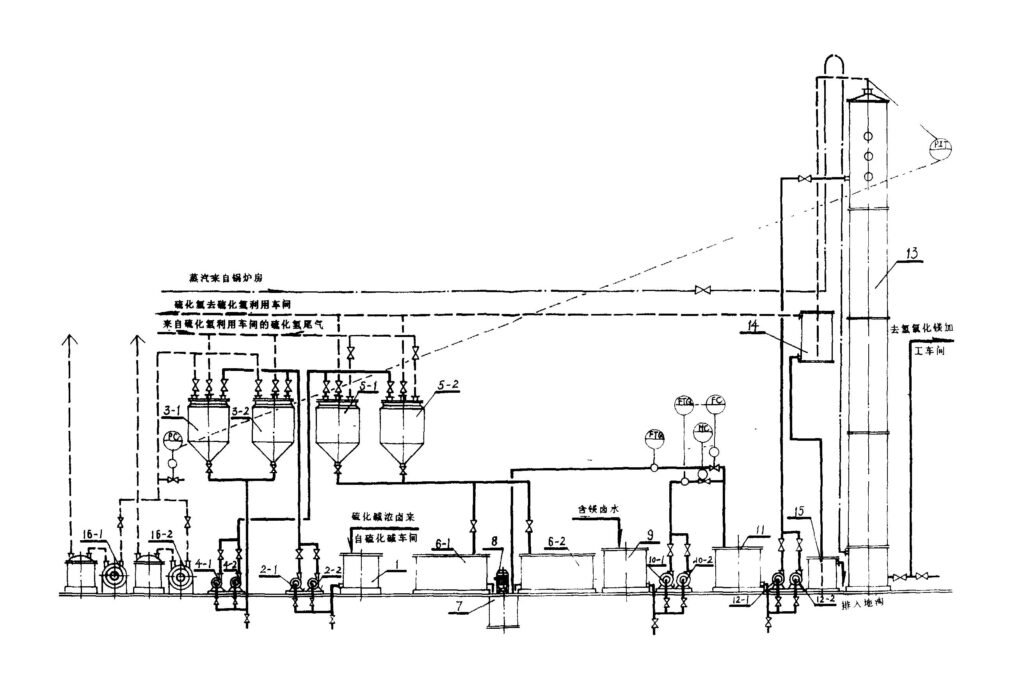
There are roughly four methods for producing magnesium hydroxide using magnesium chloride as a raw material:
(1) Lime milk method
Using MgCl2•6H2O as raw material, it reacts with milk of lime to form Mg(OH)2 precipitation. The reaction formula is:
MgCl2 + Ca(OH)2 → CaCl2 +Mg(OH)2 ↓
The advantage of this method is that the lime milk is cheap and easy to obtain, the production cost is low, and the economic benefits are good; the disadvantage is that if the process is not arranged properly, the product will have a fine particle size, even gelation, large specific surface area, and easy to absorb and wrap a large number of impurity ions. , So that its purity is low, and the filtration performance is also poor. In addition, the hydration activity of the raw material quicklime is also one of the keys to this method, and quicklime fired at high temperatures cannot be used. The too high temperature for burning quicklime or too long high-temperature residence time will result in complete quicklime crystallization and low hydration activity. On the contrary, if the raw material quicklime has many structural defects and the hydration reaction is fast, this method is advantageous. The reaction endpoint of this method is generally controlled at about pH=10.4.
(2) Ammonia method
Take MgCl2 as the raw material and ammonia water as the precipitant for the reaction. The reaction formula is:
MgCl2+2NH3•H2O → Mg(OH)2 ↓ + 2NH4Cl
The advantage of this method is that the purity of the synthesized magnesium hydroxide is high, but the disadvantage is that the yield is low and the particle size distribution is wide, especially the operating environment is harsh due to the strong volatility of ammonia, and the environmental pollution problem is prominent. In order to overcome the above shortcomings, calcium chloride is often added to the mother liquor to increase the yield of magnesium hydroxide (up to 80%); the reaction temperature is controlled at 40~50°C to obtain a product with good filtration and washing performance and higher yield. In addition, an excess of ammonia is about 20%.
(3) Sodium hydroxide method
Mg(OH)2 is prepared by reacting magnesium chloride aqueous solution with caustic soda, and the reaction formula is:
MgCl2 + 2NaOH → Mg(OH)2 ↓ + 2NaCl The process is simple to operate, and the morphology, structure, particle size, and purity of the product are easy to control, and the added value is large. It is suitable for preparing high-purity micro-products. Since sodium hydroxide is a strong base, improper conditions in this method will cause the particle size of the magnesium hydroxide to be smaller, which will bring difficulties to product performance control and filtration. Therefore, for different raw materials, the synthesis conditions must be strictly controlled.
(4) Electrolytic brine method
The reaction formula is as follows:
MgCl2 + 2NaOH → Mg(OH)2 ↓ + 2NaCl
2NaCl + 2H2O → 2NaOH + H2 ↑ + Cl2 ↑
The by-product sodium chloride is electrolyzed to prepare a precipitation agent and recycled for use in precipitation of magnesium chloride, without or less use of externally purchased caustic soda. This method is suitable for the preparation of high-purity, high-yield magnesium hydroxide, but due to its high energy consumption, it is not suitable for areas with tight electricity.
Among the above four methods, the auxiliary raw material of the lime milk precipitation method is lime milk, which is the easiest to solve and has the lowest price; the by-product CaCl2 has a wide range of uses, is easy to handle, and has the least impact on the environment. The lime milk precipitation method should be preferred for the production of environmental protection grade and flame retardant grade magnesium hydroxide. The lime milk precipitation method is generally used for the production of magnesium hydroxide using magnesium chloride as the raw material in the world.


One Response
Good luck!